Isotopes in Biology?
Every time we drink a glass of water, roughly one out of every 500 water molecules is built with a heavy 18O atom and one out of every 4,500 is built with heavy hydrogen, 2H. Drink a soy latte at breakfast? One out of every 274 of the nitrate molecules contained 15N, the heavy version of nitrogen and roughly one out of every 45,000 contained both heavy nitrogen and heavy oxygen. Accordingly, while it slides under the radar of most of our chemical and biological investigations, there is a steady stream of isotopes flowing through our body each day - most of which are heavy isotopes. Heavy isotopes vibrate at slower frequencies, they slow down chemical reactions, and they alter the spin and magnetic states of molecules. On an average day our metabolic pathways process over 10^15 heavy isotopes. Many of these will be woven into our functional molecules, enzymes, and tissues – does that affect their performance? Might problems arise if these isotopes migrate and aggregate in regions which require extreme fidelity to function properly? How changes in nuclear mass affect metabolic pathways is not well understood.
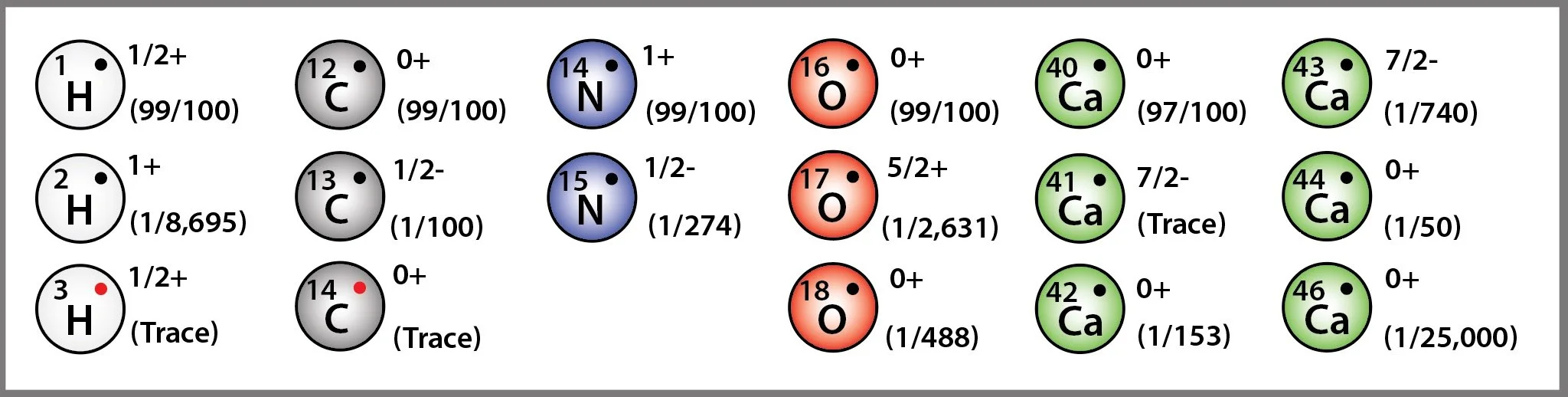
The 5 most common atoms found in a human body are shown in the diagram above, together with their mass, their nuclear spin, and their relative abundance. Although isotopes come in both light and heavy versions, a quick inspection of the chart shows how it is the lightest isotope that we tend to associate with these elements. As each new cell is built, over a trillion molecules will be united for the first time, forming partnerships and allegiances that, collectively, seek to deliver a structure that is most compatible with, or most useful to, the host. Although electron configuration dictates the bonding structure of molecules, their isotopic state will affect how those molecules perform.
This experimental program is investigating a potential new mechanism for energy regulation within cells that naturally emerges once freed from the restrictions of the B.O.A.. The intent is to find evidence for a higher frequency coupling occurring between nucleon isomeric resonance (internal to molecules) and resonance in cell electric potentials. Our initial focus is directed towards altered resonance effects caused by either heavy and light isotopes – specifical iron 58Fe & 54Fe due to the critical role it plays in cell growth, the immune system, and within enzymes regulating electron transport within mitochondria. A second focus is on altered resonance effects within iron containing structures when either heavy water (D2O) or heavy nitrogen (15N) is introduced into the cell environment.
(See Blog page for updates on this project).