INVESTIGATION INTO THE POSSIBLE NEGATIVE HEALTH EFFECTS CAUSED BY STABLE ISOTOPES WOVEN INTO TISSUES AND MEMBRANES
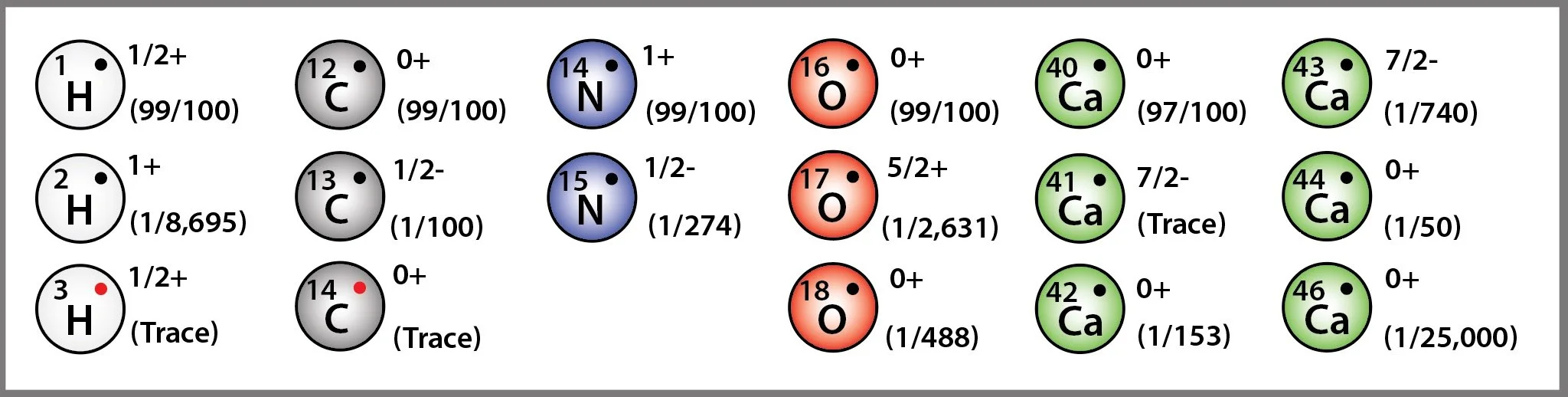
The 5 most common atoms found in a human body are shown in the diagram above, together with their mass, their nuclear spin, and their relative abundance. Every time we drink a glass of water, roughly one out of every 500 water molecules is built with a heavy 18O atom and one out of every 4,500 is built with heavy hydrogen, 2H. Drink a soy latte at breakfast? One out of every 274 of the nitrate molecules contained 15N, the heavy version of nitrogen and roughly one out of every 45,000 contained both heavy nitrogen and heavy oxygen. On an average day our metabolic pathways process over 10^15 heavy isotopes. While it slides under the radar of most of our chemical and biological investigations, there is a steady stream of isotopes flowing through our body each day - many of which will be woven into our functional molecules, enzymes, tissues, and even our DNA. Heavy isotopes form stronger bonds, they vibrate at slower frequencies, they slow down chemical reactions, and they alter the spin and magnetic states of molecules in subtle ways that can make the activation of previously obtainable chain reactions more challenging. Over long metabolic timelines (years) might slight differences in the mass or bond vibration frequency in protein and lipids migrate and aggregate within mitochondria? And, if so, would that lead to a weakening in the integrity of the membrane allowing it to leak charge and diminish the performance of the entire organelle? In a sense, isotopic effects mimic many of the characteristics we associate with aging.
Numerous research articles suggests there may be a correlation between isotope ratios and diseases. THE HEAVY ISOTOPE EXPERIMENTS will investigate their possible ill-health effects on energy molecules beginning with isotopes of nitrogen and iron woven into heme structures such as porphyrin molecules like hemoglobin in RBC’s and mitochondria.